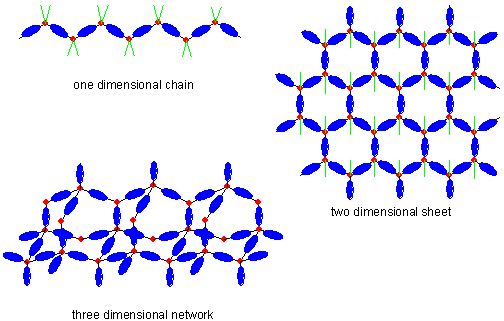
Molecular growth in one, two, or three dimensions is controlled by terminating groups (shown as green lines). Polymers that incorporate metal atoms may combine some of the desirable properties of organic polymers (strength, flexability, etc.) with the desirable properties of metals (electrical and thermal conductivity). This combination of characteristics could lead to applications as conducting plastics. It is also apparent that many of these new materials have inherent pores, which are surrounded by metal atoms. This should increase the "traffic directing" ability mentioned above, and could lead to applications as catalysts for chemical reactions.
Metal-organic networks have potential applications as catalysts for organic
reactions. The porous structure of the network can trap the substrate. Next,
the active metal center catalyzes the reaction. After the reaction is complete,
the metal catalyst can be easily removed by filtration, conserving expensive
catalyst and improving product purity.
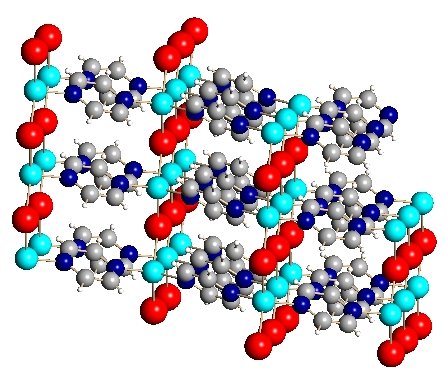
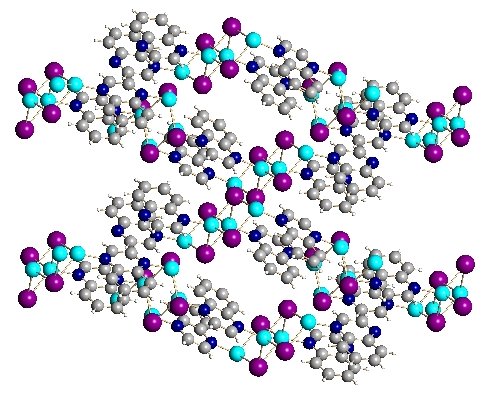
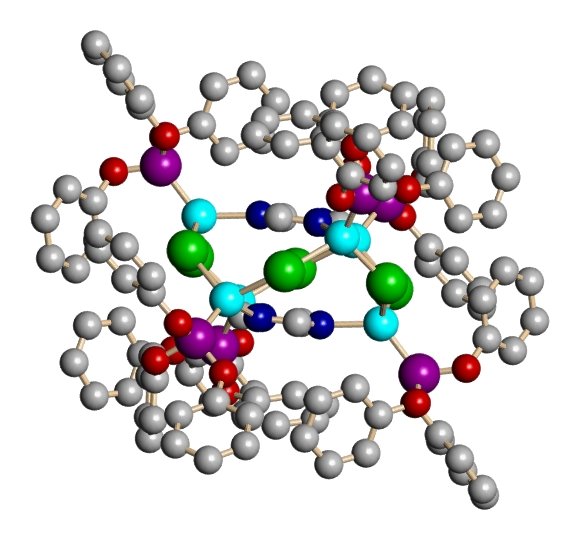
Shown below is a sampling of some of the metal-organic network compounds that our group has produced. The views are from X-ray crystallography. this technique produces a photograph-like view of the atom positions in space via diffaction of X-rays from a crystal of the compound. The atoms in light blue are copper. The large atoms in purple, red, yellow, or green are halogens (Cl, Br, I) and the structures in grey with occasional red and blue atoms are the organic linker groups.

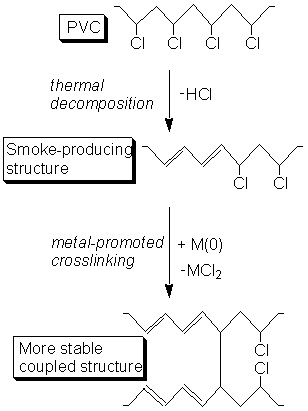
Polymer Additives. We are also exploring the used of certain metal compounds
to suppress the evolution of smoke and flame during poly(vinyl chloride) (PVC)
combustion. PVC is one of the two highest production commercial polymers worldwide.
It is used in construction and electric wiring. Although PVC itself
does not burn, its decomposition at high temperatures produces dangerous
smoke and byproducts which do burn. We are developing metal-based additives
which are designed to crosslink the polymer chains of decomposing PVC, rendering
them more stable toward decomposition. This process is illustrated
below.